Scientific Name: Citrus x limon, Citrus limon
Common Names: Lemon
Description
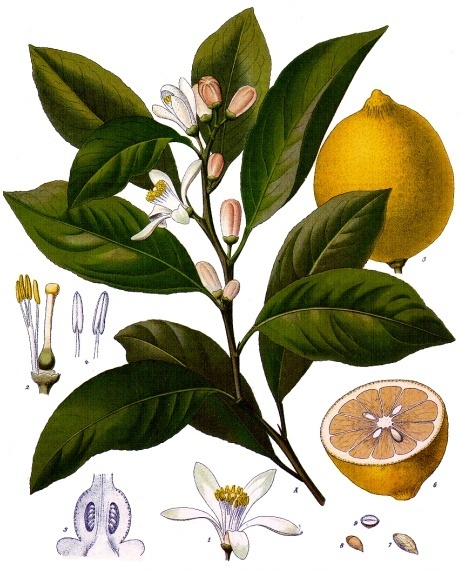
The lemon is a small evergreen tree native to Asia that belongs to the citrus family (Rutaceae). It can grow up to 6 meters high and is cultivated in warm regions such as the Mediterranean, Florida, and California. It has leathery, dark-green, ovate leaves and flowers with 4-5 petals that are purplish on the underside but white on the upper surface when open. The large, oval-shaped fruits, with a nipple-like protrusion on the end furthest from the stem, are yellow when ripe.
Culinary Uses
Lemon juice, rind, and zest from the lemon fruit are all used in many types of food and drink.
The acidic lemon juice is used to make drinks such as lemonade, soft drinks, and cocktails, and foods such as marmalade (with the rind added), meringue, and sherbert. In the UK, lemon juice is sometimes added to pancakes. Used as a marinade for fish, the acid in the juice breaks down the amines that cause "fishy odor," thereby neutralizing them by turning them into non-volatile ammonium salts. It can also be used to tenderize meat, by partially hydrolyzing its tough collagen fibers. However, the meat tends to dry out when cooked because the low pH of the lemon juice denatures its proteins. Lemon juice is also an effective short-term preservative on fruits that oxidize quickly and turn a brown color when sliced, such as apples, bananas, and avocados. The acid from the juice denatures the enzymes (proteins with a specific shape and function) that cause the browning to occur.
Lemon zest, which is the grated outer rind, is used in flavoring baked goods, rice, desserts, and so on.
Medicinal Uses, Other Practical Uses, and Chemistry
A raw lemon without the peel contains 53 mg/ 100 g of vitamin C (ascorbic acid), a water soluble vitamin important for the prevention of scurvy (a disease caused by deficiency of vitamin C) and for helping the immune system resist infection. Vitamin C is also an antioxidant and helps the body absorb iron. It is important for healthy teeth, bones and gums; the synthesis of thyroxine (a major thyroid hormone); and the metabolism of amino acids. Lemon juice, like other citrus fruits, also has a high concentration of citric acid (5-6%), which is a weak organic acid useful in cheese processing, lending an acidic tang to foods and beverages, electroplating, water conditioning, and so on.
Lemon essential oil, which comes from the peel of the fruit, is composed mainly of limonene. Other components are: γ-terpinene, α-pinene, β-pinene, terpinoline, neral (citral B), geranial (citral A), neryl acetate, geranyl acetate, trans-α-bergamotene, sabinene, (E)-caryophyllene, and β-bisabolene. Lemon oil is phototoxic, meaning it should not be used on the skin if the skin will be exposed to the sun (for up to 72 hours). Like orange oil, lemon oil can be used as a natural insect repellant.
Lemons can be used as low-powered batteries in educational science demonstrations and their juice used for acid in some classroom chemistry experiments.
For cleaning, a halved lemon fruit dipped in salt or baking soda can be used to scrub copper pots and pans. The acid dissolves the tarnish while the salt or baking soda works as an abrasive to assist scrubbing. Lemon peels, which contain lemon oil, can be used to polish wood furniture.
In some studies, the scent of lemon (lemon oil), has shown to have a positive enhancing effect on a person's mood (see "Links for More Information" on aromatherapy from Yale and NIH to learn more about the studies and their results).
Pictures
Ripe lemons (fruit)
Lemon flower
A young lemon tree with unripe fruits (green in color)
LINKS FOR MORE INFORMATION:
PLANTS Profile
Missouri Botanical Garden
Purdue Horticulture: Lemon
EOL: Encyclopedia of Life
All Recipes.Com: Lemon Recipes
Aromatherapy: Exploring Olfaction (Yale University)
Aromatherapy May Make Good Scents, But Does It Work? (NIH)
Impact of lemon oil composition on formation and stability of model food and beverage emulsions (Science Direct)


No comments:
Post a Comment